
Other Water Treatment Options
Water quality issues can vary, and depending on the substances found in your water supply, there will be a specific water treatment approach required. There are typically other water treatment options for suspended solids, for bacteria, and for dissolved solids or minerals.
Suspended Solids: This is material that exists in a suspended (insoluble) form in water, which will typically settle at the bottom of a glass or bottle as a visible sediment. This type of contamination is very common in surface waters, such as rivers where water movement generates high turbidity. To remove this type of contamination, the physical method of filtration is employed. Different filter pore sizes allow for removal of particles to specific “micron” (millionth of a meter) ratings.
There exists a variety of filter cartridges or media filters, the selection depending largely on the amount of suspended solids, and the type of water use. For example, a standard filter for drinking water is typically in the 5 to 1 micron range. The smallest particle size visible to the human eye is around 30 microns, meaning that suspended solids vary greatly from those that can be seen easily and those that cannot.
Microbiological: This type of contamination is a major challenge in many parts of the world where water distribution systems are lacking or not in a good state of operation and where water is stored for use in drought conditions. In these situations, the primary concern is to eliminate illness-causing pathogens that may be present in the water, including bacteria, viruses, and parasites. In these same areas of high heat and humidity, reproduction of harmful organisms is often very rapid.
Among the most popular technologies for the eradication of microbiological contamination is disinfection by ultraviolet light (UV). Advantages of UV treatment include immediate disinfection, broad spectrum coverage of pathogens, no chemical additions that can alter the taste and odor of the water and produce harmful disinfection by-products (DBPs). UV disinfection systems emit ultraviolet light at a wavelength of 254 nanometers, which has a powerful germicidal effect on micro-organisms in water. It is important to ensure the appropriate UV dose by employing professional installation services and system sizing. UV disinfection technology is not new but has been refined to the point where it is an excellent option for disinfection being convenient, safe and reliable.
Other methods of disinfection such as chlorine and ozone are also used for drinking water. Chlorine is widely used for its residual effect so that water remains treated throughout distribution systems. Health conscious trends have led to the desired removal of chlorine before the consumption of water not least because of its unpleasant taste and the potential health effects of DBPs. Ozone is a very powerful oxidant, having specific applications in the bottling of beverages a last step after UV disinfection to sanitize containers and add shelf life to purified water.
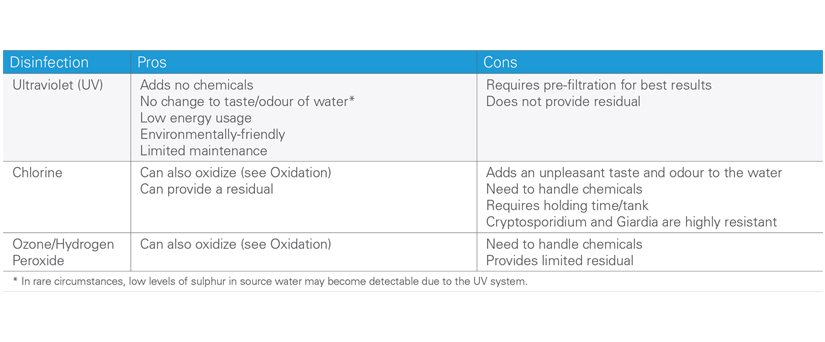
Comparison of Disinfection Options
Dissolved Solids: As the term suggests, this contaminant refers to material dissolved the water. The solids are chemically dissolved as part of the water, and their concentrations are measured overall as total dissolved solids (TDS), and the maximum concentration allowed in drinking water is usually around 1000 ppm. Total dissolved solids is a general measure of all solids dissolved while more detailed analysis will report the levels of individual dissolved species (for example, calcium, magnesium, bicarbonates, and nitrates). Dissolved solids are generally in the form of “ions”, cations carrying a positive charge and anions carrying a negative charge.
Some of the most important dissolved contaminants are arsenic, uranium, fluorides and nitrates. Special attention is given to these contaminants due to important implications for public health. Permissible limits for various dissolved contaminants vary according to the health effect. For example, acceptable levels of calcium are generally 50 thousand times higher than for arsenic. Technologies employed to remove dissolved solids include demineralization by ion exchange, distillation, and reverse osmosis.
Demineralization by Ion Exchange: This is a process by which up to 99% of dissolved minerals are removed by passing water through a bed of cationic exchange resin and then passing through an anionic resin bed. This process is able to generate a high degree of demineralized water. The first resin column removes positively charged ions while the second column removes negative ions. This technology is widely used at an industrial level. A similar process, using cationic resin only, is used in water softeners.
Distillation: This process delivers water of high purity by evaporating water, leaving the mineral content behind, followed by condensation of the resulting steam. Its application is limited by the high energy requirement.
Reverse Osmosis: A very successful process to remove dissolved solids, for residential, commercial and industrial applications. This process uses a membrane which rejects a high percentage of dissolved solids in the water. Because of the way in which this technology operates, it is important to maintain good design as it is always necessary to have a % rejection of the incoming water, resulting in two flows, one with high concentrations of dissolved solids, and another with low level of dissolved solids. It is important to select systems that water use efficient, producing more product water (permeate) and wasting as little water as possible (rejection).